Cell Publication Elucidates How SARS-CoV-2 Dramatically Rewires Important Cell Programming; Identifies Existing Therapeutics That May Fight COVID-19
For the First Time, Images Show Rapid Spread of Infection May be Explained by Extensive Filopodia, Stringy Protrusions in Virally Infected Cells
Discoveries Emanate from QBI UCSF “Blueprint Map” Revealing how SARS-CoV-2 Hijacks Human Cells
SAN FRANCISCO, June 28, 2020 (GLOBE NEWSWIRE) -- Pioneering a new paradigm in drug discovery by leveraging biological understanding of how a virus interacts with its host, an international team of researchers conducted a global analysis of proteins in human cells to determine mechanisms by which SARS-CoV-2 shifts cellular activity in infected cells. During the study, the researchers discovered for the first time that cells infected with SARS-CoV-2 exhibit filopodia, stringy arm-like extensions, which may explain rapid viral spread throughout the body.
The quantitative study, ‘The Global Phosphorylation Landscape of SARS-CoV-2 Infection’ online now in Cell, builds upon a previously published novel “blueprint” of the 332 human proteins that interact with 27 SARS-CoV-2 viral proteins. In an extension of the blueprint, the scientists evaluated all human proteins that exhibited changes in phosphorylation. Phosphorylation, the addition of a phosphoryl group to a protein by an enzyme class called kinases, plays a pivotal role in the regulation of most cell processes including the production of the cytoskeleton, protein function, cell-to-cell communication, cell growth and cell death. The scientists collaborated with Zoic Labs to overlay this new phosphorylation information onto the interactive version of the previously published protein-protein interaction map.
“By conducting a systematic analysis of the changes in cell programming through phosphorylation when SARS-CoV-2 infects a cell, we identified several key factors that will not only inform the next areas of biological study but also therapies that may be repurposed to treat patients with COVID-19,” said Nevan Krogan, Ph.D., director of the Quantitative Biosciences Institute (QBI) at the School of Pharmacy at UC San Francisco, senior investigator at Gladstone Institutes, and lead investigator of the study. Key scientists from UCSF, QBI’s Coronavirus Research Group (QCRG), Gladstone, EMBL’s European Biosciences Institute (EMBL-EBI) in Cambridge, England, Icahn School of Medicine at Mount Sinai in New York, Institut Pasteur in Paris, University of Freiburg in Germany and NIH Rocky Mountain Laboratories in Montana participated in the research.
”Our understanding of how this virus co-opts human cells continues to be a fruitful source of key virus-host cell interactions, which are leading us to promising therapies already in existence that can disrupt the virus’ clever way of surviving and thriving within the body,” continued Dr. Krogan.
Phosphorylation of Viral and Human Host Proteins During Infection
The team determined that 40 of the 332 human proteins that interact with SARS-CoV-2 were significantly differentially phosphorylated in cells infected with SARS-CoV-2. In addition, they identified 49 human kinases, out of a total of 518, that showed changes – either upregulation or downregulation – of phosphorylation activity.
The most strongly hijacked kinases include casein kinase II (CK2), kinases within the p38/MAP kinase (p38/MAPK) pathway, cyclin-dependent kinases (CDKs) and phosphatidylinositol 5-kinase (PIKFYVE), all of which fall within a set of cell signaling pathways. Because kinases possess certain structural features, they are very druggable targets with more than 500 compounds commercially available or in development.
Researchers then set out to evaluate whether existing compounds would inhibit SARS-CoV-2 in infected cells in which specific kinases had been manipulated by the virus.
“We employed state-of-the-art bioinformatics approaches to readily identify regulated kinases from sparse phosphorylation profiles, many of which are likely to be established drug targets with therapeutic potential,” commented Pedro Beltrao, Ph.D., group leader at EMBL’s European Bioinformatics Institute.
Specifically, the researchers found that:
- CK2, a kinase that plays a key role in cytoskeleton formation, cell growth and proliferation as well as apoptosis (cell death), physically interacted with the SARS-CoV-2 viral N protein and was significantly more activated in virally infected cells. Using a CK2 inhibitor in the laboratory experiments eliminated the virus.
- Several kinases within the p38/MAPK pathway respond to and control the production of potentially harmful pro-inflammatory cytokines including IL-6, IL-10 and TNF-alpha. Inhibiting p38/MAPK signaling in virally infected suppressed the overproduction of inflammatory cytokines and directly impaired viral replication, suggesting that p38/MAPK inhibition may target multiple mechanisms related to COVID-19 pathogenesis.
- CDKs are kinases that regulate cell cycle and DNA damage response. Cells infected with SARS-CoV-2 have significantly reduced CDK activities, which may facilitate viral replication. Inhibition of CDKs may halt viral replication.
- PIKFYVE, a FYVE finger-containing phosphoinositide kinase that regulates cytoskeleton function, is targeted in a variety of different cancers. The compound apilimod that targets this kinase potently inhibited SARS-CoV-2 in the laboratory setting.
Repurposing Kinase Inhibitors for Treatment of COVID-19
The team triangulated changes in phosphorylation to specific protein kinase targets and identified 87 U.S. Food and Drug Administration (FDA) approved drugs and compounds in clinical trials or development. Based on an initial systematic review of these compounds, the researchers identified seven agents, primarily anti-cancer and anti-inflammatory compounds, that demonstrated antiviral activity in laboratory experiments: silmitasertib, gilteritnib, MAPK13-IN-1, SB203580, ralimetinib, apilimod and dinaciclib.
“We are encouraged by our findings that drugs targeting differentially phosphorylated proteins inhibited SARS-CoV-2 infection in cell culture,” said Kevan Shokat, Ph.D., professor in the department of Cellular and Molecular Pharmacology at UC San Francisco (UCSF). “We expect to build upon this work by testing many other kinase inhibitors while concurrently conducting gene knockout experiments with RNAi technologies to continue to identify both the underlying pathways and additional potential therapeutics that may intervene in COVID-19 effectively.”
Importantly, additional studies are needed to further characterize the anti-viral potential of the compounds identified through this analysis.
Filopodia on Virally Infected Cells Newly Discovered Through Advanced Imaging
Interestingly, while studying the impact of SARS-CoV-2 on CK2, high resolution imaging of virally infected cells produced by the NIH/NIAID/Rocky Mountain Laboratories and University of Freiburg revealed actin-rich filopodia containing viral proteins. Human CK2 and the viral N protein were found co-localized within the filopodia, suggesting that SARS-CoV-2 hijacks CK2 and co-opts it into creating these tentacle-like protrusions that poke holes in their neighboring cells.
Conversely, other viruses including vaccinia, Ebola and Marburg take over the host cell cytoskeleton to promote egress and rapid cell-to-cell spread. However, in SARS-CoV-2 infected cells, the filopodia exhibit longer tentacles and branches, enabling more aggressive transmission than some other viral infections.
“The distinct visualization of the extensive branching of the filopodia once again elucidates how understanding the biology of virus-host interaction can illuminate possible points of intervention in the disease,” continued Dr. Krogan. “Our data driven approach for drug discovery has identified a new set of drugs that have great potential to fight COVID-19, either by themselves or in combination with other drugs, and we are excited to see if they will help end this pandemic.”
About QBI: The Quantitative Biosciences Institute (QBI) is a University of California organized research unit reporting through the UCSF School of Pharmacy. QBI fosters collaborations across the biomedical and the physical sciences, seeking quantitative methods to address pressing problems in biology and biomedicine. Motivated by problems of human disease, QBI is committed to investigating fundamental biological mechanisms, because ultimately solutions to many diseases have been revealed by unexpected discoveries in the basic sciences. Learn more at qbi.ucsf.edu.
About UCSF: The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. UCSF Health, which serves as UCSF’s primary academic medical center, includes top-ranked specialty hospitals and other clinical programs, and has affiliations throughout the Bay Area. Learn more at ucsf.edu or see our Fact Sheet.
About Gladstone Institutes: To ensure our work does the greatest good, Gladstone Institutes focuses on conditions with profound medical, economic, and social impact—unsolved diseases. Gladstone is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. It has an academic affiliation with UC San Francisco. Learn more at gladstone.org.
Authorship and funding: This work was funded by grants from the National Institute of Mental Health and the National Institute of Allergy and Infectious Diseases, both part of the National Institutes of Health; the Defense Advanced Research Projects Agency; the Center for Research for Influenza Pathogenesis; the Centers of Excellence for Influenza Research and Surveillance of the National Institute of Allergy and Infectious Diseases; the Centers of Excellence for Integrative Biology of Emerging Infectious Diseases of the Agence Nationale de la Recherche (France); F. Hoffmann-LaRoche AG; Vir Biotechnology, Centre for Integrative Biological Signalling Studies (CIBSS), European Research Council (ERC) and the Ron Conway Family. Shokat is a Howard Hughes Medical Institute investigator. A complete list of authors and full funding information is available in the Cell paper.
Media Contacts
UCSF QBI
Angela Bitting, Wheelhouse LSA
a.bitting@comcast.net
925-202-6211
UCSF
Pete Farley, UCSF Office of Communications
peter.farley@ucsf.edu
415-317-3781
Sylvia Wheeler, Wheelhouse LSA
swheeler@wheelhouselsa.com
Gladstone Institutes
Megan McDevitt, Gladstone Communications
megan.mcdevitt@gladstone.ucsf.edu
415-734-2019
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/9145af6f-0c38-4690-97a7-d60db05a6770
https://www.globenewswire.com/NewsRoom/AttachmentNg/96f65f4c-c1a8-4ed2-a504-a48019388204
https://www.globenewswire.com/NewsRoom/AttachmentNg/c3198fcf-1b2a-4bd0-a783-4d9026327c19
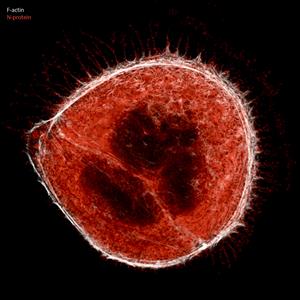
Caco-2 cells
Fluorescence microscopy image of a two cell cluster of Caco-2 cells (human colon cells) infected with SARS-CoV-2 virus, the causative agent of COVID-19. Infected cells produce filopodia protrusions (white) extending out from the cell surface containing viral particles (M protein in red). Photo credits to Dr. Robert Grosse, CIBSS, University of Freiburg.
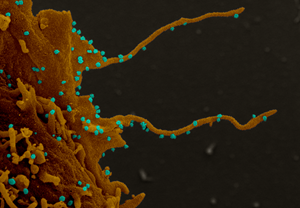
Vero Cells
Electron microscopy image of Vero E6 cells infected with SARS-CoV-2 virus, the causative agent of COVID-19, produce filopodia protrusions (finger-like projections) extending out from the cell surface to enable budding of viral particles (circular spheres) and infection of nearby cells. Photo credits to Dr. Elizabeth Fischer, NIAID/NIH.
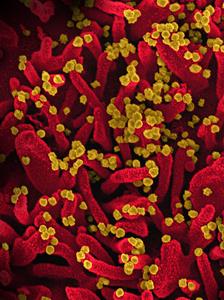
Vero Cells
Electron microscopy image of Vero E6 cells infected with SARS-CoV-2 virus, the causative agent of COVID-19, produce filopodia protrusions (red) extending out from the cell surface to enable budding of viral particles (yellow) and infection of nearby cells. Photo credits to Dr. Elizabeth Fischer, NIAID/NIH. Bouhaddou et al. © Elsevier 2020
Compra paquetes de fotos y videos a precios flexibles
Para pagos desde Argentina, tomamos el precio a la cotización del día del dólar oficial del Banco Central de la República Argentina
- 30 Videos - U$D 600.00
- 100 Videos - U$D 1800.00
- 400 Videos - U$D 5600.00
- 50 Fotos + 30 Vídeos - U$D 900.00
- 100 Fotos + 100 Videos - U$D 2250.00
- 500 Fotos + 400 Videos - U$D 6230.00